
NEWS
Catalytic Converters - Lean NOx Trap
Another type of catalyst being developed for diesel engines are known as lean NOx traps (LNT) because they function by trapping the NOx in the form of a metal nitrate during lean operation of the engine. The most common compound used to capture NOx is Barium Hydroxide or Barium Carbonate. Under lean air to fuel operation, NOx reacts to form NO2 over a platinum catalyst followed by reaction with the Barium compound to form BaNO3. Following a certain amount of lean operation, the trapping function will become saturated and must be regenerated. This is commonly done by operating the engine in a fuel rich mode for a brief period of time to facilitate the conversion of the barium compound back to a hydrated or carbonated form and giving up NOx in the form of N2 or NH3. LNT catalyst can be combined with a zeolite based SCR catalyst to trap ammonia and further reduce NOx via a selective catalytic reduction reaction to nitrogen.
Catalytic Converters - Non-Selective Catalytic Reduction System
A non-selective catalytic reduction system (NSCR) utilizes a precious-metal based catalytic converter, similar in design to the three-way catalytic converters used on cars and trucks, to reduce NOx, unburned HC, and CO. NSCR systems are currently applied to IC engines with fuel-rich ignition systems. For proper operation of a NSCR system, the combustion process must occur with an air/fuel ratio slightly fuel-rich of stoichiometry. In the presence of the catalyst under this condition, NOx is reduced by the CO, resulting in nitrogen and carbon dioxide. Typical NOx conversion ranges from 80 to 95 percent with corresponding decreases in CO and HC.
Catalytic Converters - Three-Way Catalytic Converter
The three-way converter (TWC) has been the primary emission control technology on light-duty gasoline vehicles since the early 1980s. The use of TWCs, in conjunction with an oxygen sensor-based, closed-loop fuel delivery system, allows for simultaneous conversion of the three criteria pollutants, HC, CO, and NOx, produced during the combustion of fuel in a spark-ignited engine. The active catalytic materials are present as a thin coating of precious metal (e.g., Pt, Pd, Rh), and oxide-based inorganic promoters and support materials on the internal walls of the honeycomb substrate. The substrate typically provides a large number of parallel flow channels to allow for sufficient contacting area between the exhaust gas and the active catalytic materials without creating excess pressure losses. Although the primary components and function of a TWC has remained relatively constant during its more than twenty years of use on light-duty gasoline vehicles, each of the primary converter components (catalytic coating, substrate, mounting materials) has gone through a continuous evolution and redesign process in order to improve the overall performance of the converter while maintaining a competitive cost effectiveness of the complete assembly.

Catalytic Converters - Diesel Oxidation Catalyst
In most applications, a diesel oxidation catalyst consists of a stainless steel canister that contains a honeycomb structure called a substrate or catalyst support. There are no moving parts, just large amounts of interior surface area. The interior surfaces are coated with catalytic metals such as platinum or palladium. It is called an oxidation catalyst because the device converts exhaust gas pollutants into harmless gases by means of chemical oxidation. In the case of diesel exhaust, the catalyst oxidizes CO, HCs, and the liquid hydrocarbons adsorbed on carbon particles. In the field of mobile source emission control, liquid hydrocarbons adsorbed on the carbon particles in engine exhaust are referred to as the soluble organic fraction (SOF) -- the soluble part of the particulate matter in the exhaust. Diesel oxidation catalysts are efficient at converting the soluble organic fraction of diesel particulate matter into carbon dioxide and water.
Oxidation catalyst retrofits have proven effective at reducing particulate and smoke emissions on older vehicles. Under the U.S. EPA's urban bus rebuild/retrofit program, five manufacturers certified diesel oxidation catalysts as providing at least a 25 percent reduction in PM emissions for in-use urban buses. Certification data also indicates that oxidation catalysts achieve substantial reductions in CO and HC emissions. Currently, under the ARB and EPA retrofit technology verification processes, several technology manufacturers have verified diesel oxidation catalysts as providing at least a 25 percent reduction in PM emissions.
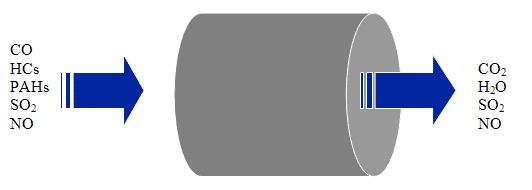
Catalytic Converters - SCR System
A Selective Catalytic Reduction (SCR) system uses a metallic or ceramic wash-coated catalyzed substrate, or a homogeneously extruded catalyst and a chemical reductant to convert nitrogen oxides to molecular nitrogen and oxygen in oxygen-rich exhaust streams like those encountered with diesel engines. In mobile source applications, an aqueous urea solution is usually the preferred reductant. Upon thermal decomposition in the exhaust, urea decomposes to ammonia which serves as the reductant. In some cases ammonia has been used as the reductant in mobile source retrofit applications. As exhaust and reductant pass over the SCR catalyst, chemical reactions occur that reduce NOx emissions to nitrogen and water. SCR catalysts can be combined with a particulate filter for combined reductions of both PM and NOx.
Open loop SCR systems can reduce NOx emissions by 75 to 90 percent. Closed loop systems on stationary engines can achieve NOx reductions of greater than 95 percent. SCR systems are also effective in reducing HC emissions up to 80 percent and PM emissions 20 to 30 percent. Like all catalyst-based emission control technologies, SCR performance is enhanced by the use of low sulfur fuel.
Catalytic Converters - Lean NOx Catalyst
Controlling NOx emissions from a diesel engine is inherently difficult because diesel engines are designed to run lean. In the oxygen-rich environment of diesel exhaust, it is difficult to chemically reduce NOx to molecular nitrogen. The conversion of NOx to molecular nitrogen in the exhaust stream requires a reductant (HC, CO or H2) and under typical engine operating conditions, sufficient quantities of reductant are not present to facilitate the conversion of NOx to nitrogen.
Some lean NOx catalyst (LNC) systems inject a small amount of diesel fuel or other reductant into the exhaust upstream of the catalyst. The fuel or other hydrocarbon reductant serves as a reducing agent for the catalytic conversion of NOx to N2. Other systems operate passively without any added reductant at reduced NOx conversion rates. A lean NOx catalyst often includes a porous material made of zeolite (a micro-porous material with a highly ordered channel structure), along with either a precious metal or base metal catalyst. The zeolites provide microscopic sites that are fuel/hydrocarbon rich where reduction reactions can take place. Without the added fuel and catalyst, reduction reactions that convert NOx to N2 would not take place because of excess oxygen present in the exhaust. Currently, peak NOx conversion efficiencies typically are around 10 to 30 percent (at reasonable levels of diesel fuel reductant consumption).
